
Researchers and teachers worldwide took this opportunity to reflect on the importance of the periodic table and spread awareness about it in classrooms and beyond. UNESCO named 2019 the International Year of the Periodic Table to mark the 150 th anniversary of Mendeleev’s publication.
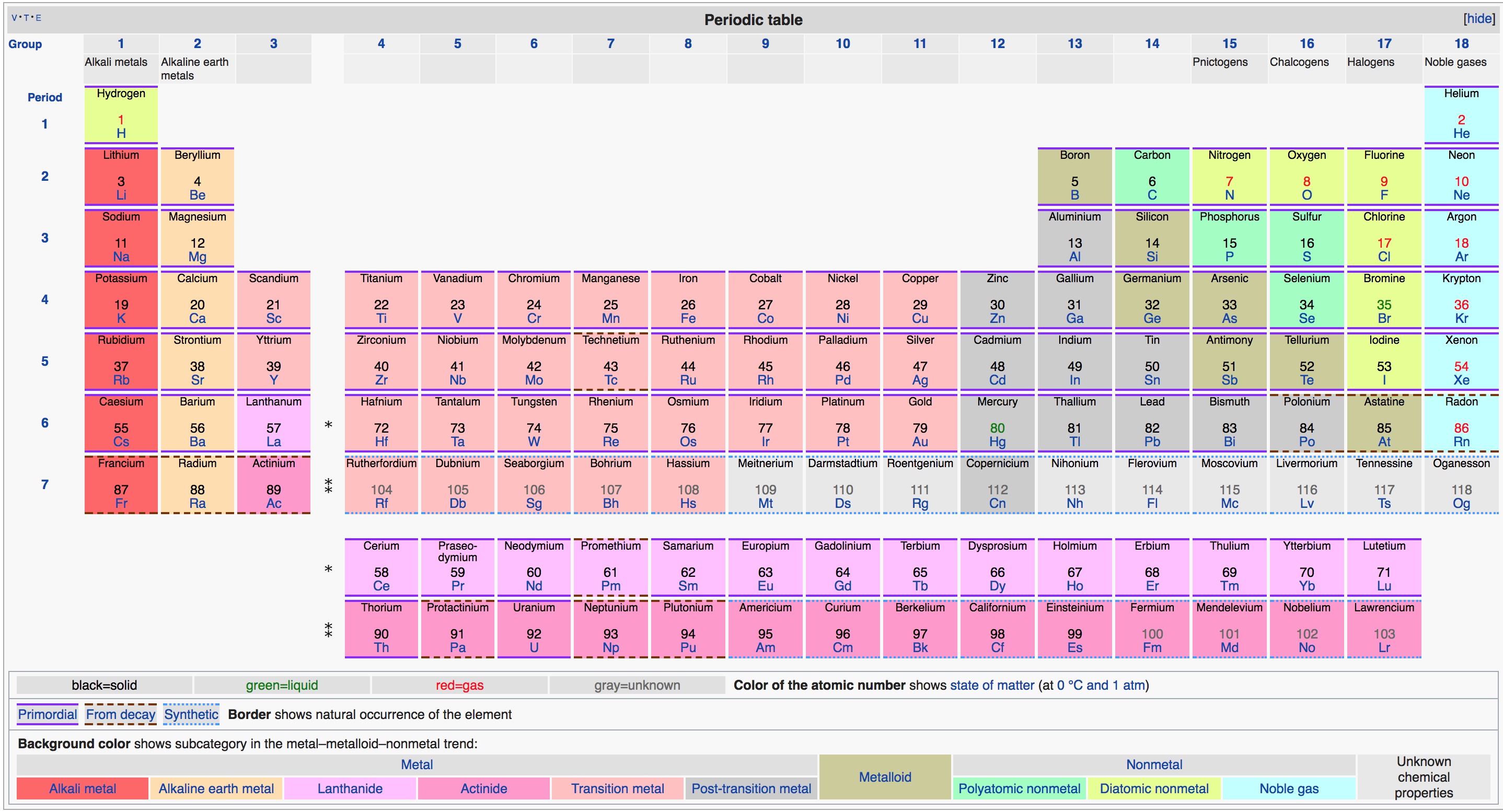
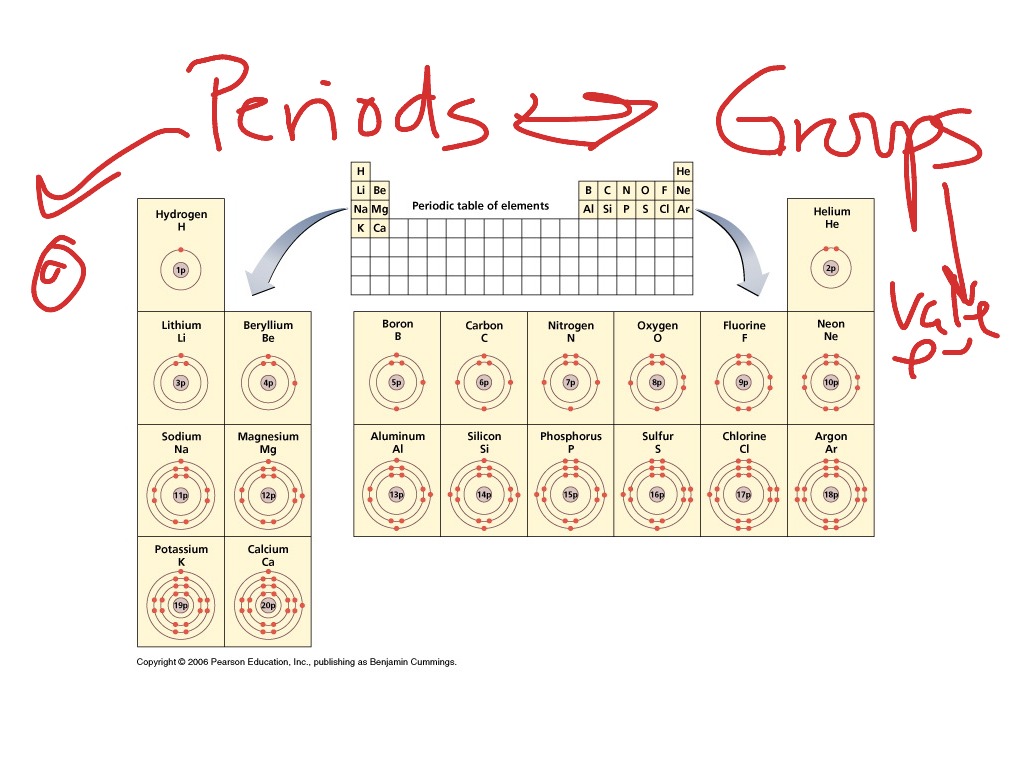
It is used to develop chemicals used in the pharmaceutical and cosmetics industries and batteries used in technological devices. Scientists use the table to study chemicals and design experiments. The periodic table provides information about the atomic structure of the elements and the chemical similarities or dissimilarities between them. The vertical columns, called groups, consist of elements with similar chemical properties. In the periodic table, the horizontal rows are called periods, with metals in the extreme left and nonmetals on the right. This helped explain disparities in earlier versions that had used atomic masses.
He then rearranged the elements in the periodic table on the basis of atomic numbers. In 1913, English physicist Henry Moseley used X-rays to measure the wavelengths of elements and correlated these measurements to their atomic numbers.

The concept of sub-atomic particles did not exist in the 19 th century. based on their atomic weights and chemical similarities.". The 1869 periodic table by Mendeleev in Russian, with a title that translates "An experiment on a system of elements. In 1955 the 101st element was named mendelevium in his honor. The later discovery of elements predicted by Mendeleev, including gallium (1875), scandium (1879) and germanium (1886), verified his predictions and his periodic table won universal recognition. The Royal Society of London awarded the Davy Medal in 1882 to both Mendeleev and Meyer. He left gaps for undiscovered elements but never predicted their properties. German chemist Lothar Meyer produced a version of the periodic table similar to Mendeleev’s in 1870. Some discrepancies remained the position of certain elements, such as iodine and tellurium, could not be explained. Later eka-aluminium was discovered as gallium. Mendeleev predicted the properties of some undiscovered elements and gave them names such as "eka-aluminium" for an element with properties similar to aluminium. While arranging the elements according to their atomic weight, if he found that they did not fit into the group he would rearrange them. In 1869, Russian chemist Dmitri Mendeleev created the framework that became the modern periodic table, leaving gaps for elements that were yet to be discovered. He arranged the elements in eight groups but left no gaps for undiscovered elements. He found that every eight elements had similar properties and called this the law of octaves.

For example, carbon, being12 times heavier than hydrogen, would have an atomic weight of 12.īritish chemist John Newlands was the first to arrange the elements into a periodic table with increasing order of atomic masses. They concluded that hydrogen would be assigned the atomic weight of 1 and the atomic weight of other elements would be decided by comparison with hydrogen. He arranged them in groups of three in increasing order of atomic weight and called them triads, observing that some properties of the middle element, such as atomic weight and density, approximated the average value of these properties in the other two in each triad.Ī breakthrough came with the publication of a revised list of elements and their atomic masses at the first international conference of chemistry in Karlsruhe, Germany, in 1860. Forty years later, German physicist Johann Wolfang Döbereiner observed similarities in physical and chemical properties of certain elements. In 1789, French chemist Antoine Lavoisier tried grouping the elements as metals and nonmetals. Among the scientists who worked to created a table of the elements were, from left, Antoine Lavoisier, Johann Wolfang Döbereiner, John Newlands and Henry Moseley.


 0 kommentar(er)
0 kommentar(er)
